CAR-T cell therapy brings hope for affordable Cancer tretment: First patient ‘cancer-free’
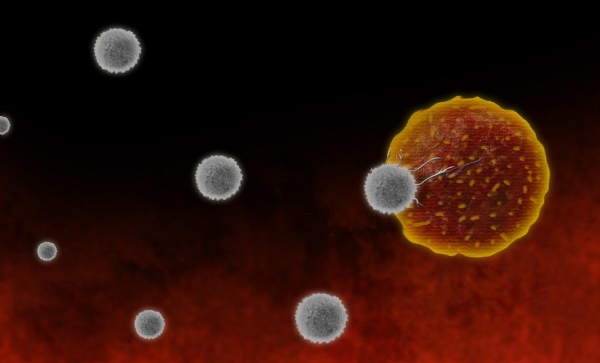
CAR-T cell therapy is a revolutionary form of immunotherapy that harnesses the power of a patient’s own immune system to fight cancer. It involves genetically modifying a patient’s T cells (a type of white blood cell) to recognize and destroy specific cancer cells. Here’s how it works:
Total Views |
In a signifcant development, a month after India’s drug regulator Central Drugs Standard Control Organisation (CDSCO), approved the commercial use of CAR-T cell therapy, now a first patient who was under going through the CAR-T cell therapy has been declared as ‘cancer free’.
As per the reports of Indian Express, the therapy has turned out to be a lifesaver for many patients, including for Dr (Col) V K Gupta, a Delhi-based gastroenterologist with 28 years of experience of working in the Indian Army. He accessed the therapy by paying just INR 42 lakh or $50,000, whereas a similar therapy costs up to INR 4 crore $480,000 abroad.
Doctors at the Tata Memorial Hospital, where Gupta underwent the procedure, said he is “currently free of cancer cells.” Gupta is the first patient to have achieved this status, something he could only dream of just a year ago.
Indigenous CAR-T cell therapy in India
— Civil Learning (@CivilLearning1) February 9, 2024
India has made significant progress in developing its own indigenous CAR-T cell therapy. In October 2023, the Central Drugs Standard Control Organization (CDSCO), India's drug regulator, approved the commercial use of NexCAR19, the first… pic.twitter.com/XJsRIsoEtE
The doctor who performed the therapy on Gupta said he was totally free of cancer cells. “While it’s premature to claim a lifelong cure, the patient is currently free of cancer cells,” Dr Hasmukh Jain, hemato-oncologist and associate professor at the Advanced Centre for Treatment, Research and Education in Cancer (ACTREC), Tata Memorial Centre, was quoted as saying by the Indian Express.
Indigenous CAR-T cell therapy in India
India has made significant progress in developing its own indigenous CAR-T cell therapy. In October 2023, the Central Drugs Standard Control Organization (CDSCO), India's drug regulator, approved the commercial use of NexCAR19, the first indigenously developed CAR-T cell therapy.
NexCAR19 is developed by ImmunoACT, a company incubated at the Indian Institute of Technology Bombay (IITB) and Tata Memorial Hospital. It is designed to treat B-cell cancers, such as leukemia and lymphoma.
It should be noted that NexCAR19 is developed by ImmunoACT, a company incubated at the Indian Institute of Technology Bombay (IITB) and Tata Memorial Hospital. It is designed to treat B-cell cancers, such as leukemia and lymphoma.
What is CAR-T Cell Therapy
CAR-T cell therapy is a revolutionary form of immunotherapy that harnesses the power of a patient’s own immune system to fight cancer. It involves genetically modifying a patient’s T cells (a type of white blood cell) to recognize and destroy specific cancer cells. Here’s how it works:
The Process
- Isolation: T cells are extracted from the patient’s blood through a process called apheresis.
- Modification: In a laboratory, the T cells are engineered to express a chimeric antigen receptor (CAR) on their surface. This CAR is designed to recognize a specific antigen (marker) present in the cancer cells.
- Expansion: The modified T cells, now called CAR-T cells, are multiplied in large numbers in the lab.
- Infusion: The expanded CAR-T cells are infused back into the patient’s bloodstream through an intravenous line.
Destroying Cancer Cells
Once infused, the CAR-T cells circulate through the body and bind to the specific antigen on the cancer cells. This binding triggers a powerful immune response:
Advantages of CAR-T Cell Therapy
- Targeted therapy: Unlike traditional chemotherapy or radiation, CAR-T cells specifically target cancer cells, minimizing harm to healthy tissues.
- Living drug: Unlike conventional drugs, CAR-T cells persist in the body, providing long-term protection against cancer relapse.
- Personalized approach: The therapy is tailored to each patient’s specific cancer, making it highly effective in certain cases.
Why it is sigificant development?
The development of indigenous CAR-T cell therapy in India is a significant achievement, as it has the potential to make this life-saving treatment more affordable and accessible to patients in India and other developing countries. Currently, CAR-T cell therapy is very expensive, costing hundreds of thousands of dollars in the United States. NexCAR19 is expected to be significantly cheaper, costing around $50,000.
Challenges of indigenous CAR-T cell therapy in India
Manufacturing: Manufacturing CAR-T cell therapy is a complex and expensive process. India will need to invest in infrastructure and expertise to ensure that it can produce enough CAR-T cells to meet the demand.
Reimbursement: Insurance companies in India may not yet cover the cost of CAR-T cell therapy. This could make it difficult for some patients to afford treatment.
Awareness: Many people in India are not aware of CAR-T cell therapy. There is a need to raise awareness of this treatment option so that more patients can benefit from it.
Overall, the development of indigenous CAR-T cell therapy in India is a positive development that has the potential to make this life-saving treatment more affordable and accessible to patients in India and other developing countries. However, there are some challenges that need to be addressed before CAR-T cell therapy can become widely available in India

